Yes. Most quantum states are evenly spaced, for example the energy levels of a "particle in a box" (i.e., the x-y-z momentum degrees of freedom) are spaced quite closely in a macroscopic 'box', leading to a classical continuum: the ideal gas.
Rotation and vibration work similarly.
These states are derived using "ladder operators", so that you climb from one state to the next, with a given amount of energy (that's approximated as constant) per step.
This breaks down in certain limits, but is useful for a first-pass approximation. Example: at high vibrational energy levels, the potential binding a molecule together, drops off. This is a consequence of the potential being, not parabolic, but sharp on one side (atoms repel each other strongly as their inner orbitals are pushed together, generating additional force) and asymptotic on the other (the potential holding the atoms together is limited, and drops to zero at large distances).
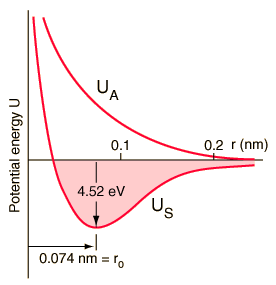
The potential well becomes wider and shallower towards the top, causing energy levels to be spaced more closely together; eventually the energy between levels is negligible, and the quantum number (corresponding to any given level) becomes hard to pin down -- because amounts of energy much greater than this are being tossed around. The result is the atom slipping out of place from time to time: dissociation, giving rise to free radicals, or ions (depending on other properties of the atoms).
Dissociation and ionization is another degree of freedom, so the heat capacity goes up again around this range; this is the phase transition from gas to plasma.
At low temperatures, there isn't enough energy to climb the first one or few energy levels of vibration on average, so the heat capacity contribution is negligible.
For more complex molecules, with weaker bonds, this can occur at lower temperatures, and you get decomposition products: charring, combustion, destructive distillation, etc. Which is of course very useful to the chemist, when it proceeds in an orderly manner.

Tim